Why healthcare for women continues to fail them
The Depo-Provera lawsuit over the potential risk of developing brain tumors is the latest failure for women’s healthcare.
By Michaela Ely
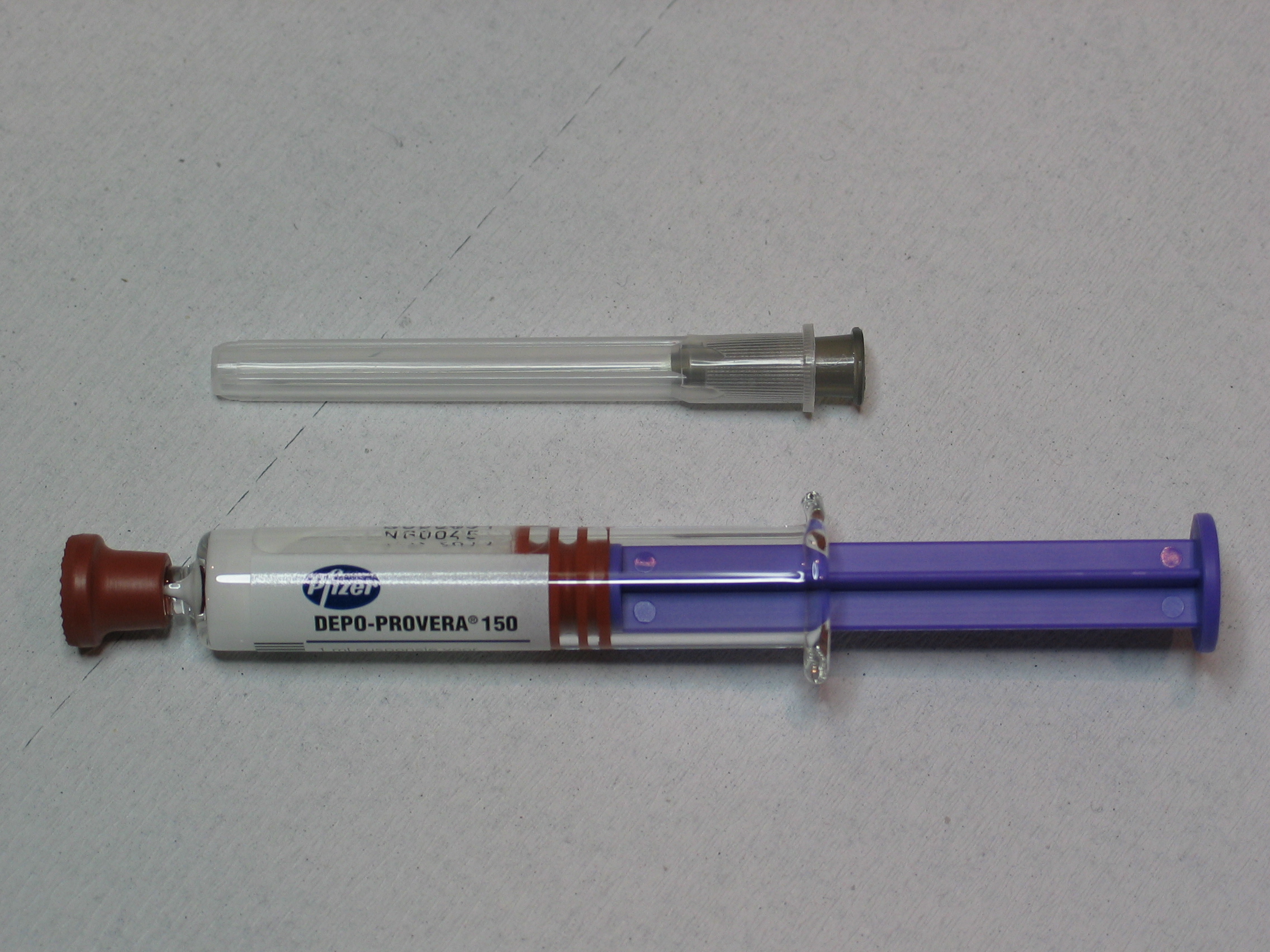
The FDA approved a new safety warning for Pfizer’s Depo-Provera injection on Dec. 12 of last year to highlight the potential risk of meningioma, a type of brain tumor. The warning states that long term usage of this injection is associated with an increased risk of developing meningioma. Meningiomas are usually non-cancerous, but they can be harmful depending on the size and location of the tumor.
Depo-Provera is a form of birth control that consists of a progestin injection that is given to a patient every three months. Progestin is a synthetic version of the hormone, progesterone. According to the CDC, one in four sexually active women in the U.S. have used Depo-Provera.
Pfizer is currently facing a lawsuit from over 1,000 women, stating that Pfizer was aware of the risk that Depo-Provera posed, pointing back to studies from 1983 that detailed the link between the injection and meningiomas. The studies that prompted Pfizer to seek out an FDA warning were published in 2024 and 2025.
The initial warning submitted by Pfizer in February 2024, which also included two pills containing medroxyprogesterone acetate (MPA), was denied by the FDA, saying that the “the findings of the available observational studies alone do not support the addition of a warning on Meningioma risk to medroxyprogesterone acetate (MPA)-containing products,” according to NBC News. Pfizer submitted a revised version of the warning in June 2025.
According to NBC News, a court filing stated that Pfizer became aware of a possible link of Depo-Provera to the risks of meningioma in 2023.
Regardless of whether or not Pfizer had the intention to deceive those who received Depo-Provera, this situation begs the question: how does a form of birth control with the severity of risks associated with it stay popular enough that one in four sexually active women use it?
The risk of meningioma is not the only risk associated with Depo-Provera; it is also linked to bone density loss for those who have been on it for two or more years. Any good healthcare provider should warn you about those risks, particularly bone density loss, as it has been a known risk since 2004, according to a study in the Contraception journal.
“I think that if [birth control is] directly linked to something known to be life ending harmful, then the medication harms likely outweighs the benefits,” UWT graduate student Riley Miller told The Ledger. “As a result, I think medication like that should be pulled from circulation and reworked to prevent this problem before getting put back on the shelves. Because there are so many types of birth control, it seems unsafe to just leave this one without being reworked.”
According to the CDC, from survey results between 2015 and 2017, approximately 65 percent of women ages 15-49 in the U.S. used some form of birth control. Even though this data is outdated, birth control is still something that impacts women throughout the U.S. and around the world.
Depo-Provera has been approved for contraceptive uses in the U.S. since 1992, but it was first approved for other uses in 1969. For a medication to have been around for over 50 years, and for its risks not to be discovered until decades after its initial use is disheartening at best. Healthcare for women should help women. It should not give them a new laundry list of issues that they might need to worry about, and it should not give them new problems to solve.